Product updates and release notes
Stay up to date with the latest improvements to FACTS. Explore new features, enhancements, and bug fixes designed to make your trial design, simulation, and analyses more powerful and efficient. See what’s new and how it impacts your workflow. For full release notes, visit our FACTS Knowledge Hub.

Introducing FACTS 8.0
Berry Consultants is delighted to announce that FACTS 8.0 has been released! FACTS users can now:
- Design and simulate phase 2 and 3 trials with an Ordinal endpoint (modules for trials with continuous, dichotomous and time to event endpoints were already available). When designing an ordinal endpoint trial with FACTS, Bayesian analysis is available with a cumulative logistic or Dirichlet (using utility values for the outcomes) models, and frequentist analysis using Wilcoxon, Proportional Odds, a t-test on utility scores or a dichotomized test.

- Using the Bayesian cumulative logistic, a full range of Bayesian dose-response models are available for multi-arm trials.
- Many of the usual features and options of FACTS simulation are available: accrual, dropouts, interims, interim stopping, arm dropping, response adaptive allocation, along with a comprehensive output of the details of the simulated trials, in a form suitable for post-processing.
- As with all FACTS designers, a Design Report describing the finished design can be generated as a Word document.
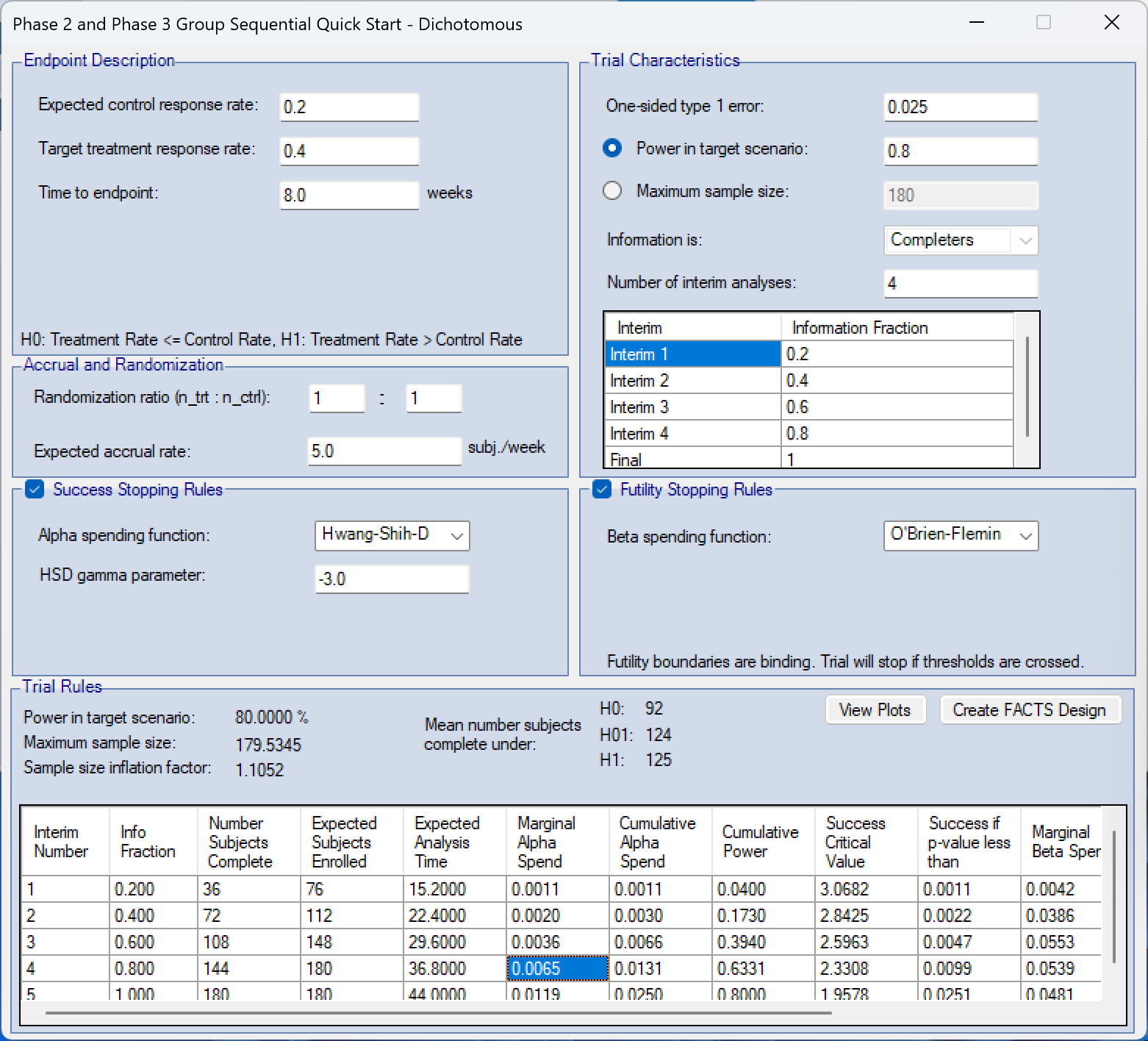
- Use Quick Start options for creating Fixed and Group Sequential Trials. Entering the typical minimal information for these trial types on a single screen. A “Create FACTS Design” button allows the user to take the trial they’ve specified and have a FACTS file created ready for simulation.
- A Quick Start generated fixed trial can be used as both a “straw man design” for comparing a more complex design to, it can also be used as the starting point for that more complex design.
- Quick Start generated group sequential trial allows detail simulations of a group sequential trial to be easily and quickly generated. These simulations allow more detailed understanding of the design and clearer communication to non-statisticians.

- FACTS 8.0 now allows for more complex Success or Futility conditions, by allowing criteria to be combined using bracketed logical AND and OR expressions.

- And lastly, as always, FACTS 8.0 is backwards compatible with previous versions of FACTS. With a couple of exceptions:
- Designs requiring a “minimum information” at an interim for a decision to be taken, will need to use the new Operational Quantities of Interest within the decision criterion itself.
- Designs where the default QOIs have been specified to be absolute tests (rather than comparisons to control) now have the default Bayesian comparison to Control QOI converted to a Bayesian absolute comparison to 0.
For more information and the full release notes, please visit the FACTS Knowledge Hub.

FACTS 7.2 Adds New Dose Escalation Methods in Latest Update
Berry Consultants announces a significant upgrade to FACTS (Fixed and Adaptive Clinical Trials Simulator). Users can now design and compare 3+3, BOIN, mTPI-2, i3+3 and CRM (using BLRM) designs. This means FACTS has the most comprehensive suite of dose escalation designs on the market. The CRM includes a rich set of additional features such as back-filling, open enrollment, and simultaneous assessment of efficacy and toxicity.

FACTS supports simulation guided clinical trial design with its unmatched simulation speed and powerful visualisations. FACTS supports trial designers in communicating with all stakeholders from an initial strawman design to the final design ready to be used.
A crucial attribute of FACTS is the recording of every simulation, both allowing the properties of a design to be reviewed and communicated in detail, and the post processing of simulations for deep analysis and exploring “what-if” questions.

This update reinforces FACTS as the leading integrated tool for fast, efficient, accurate, and innovative clinical trial design and simulation.
For more information and the full release notes, please visit the FACTS Knowledge Hub or email the FACTS team at: facts@berryconsultants.com


We are delighted to announce the release of FACTS 7.1.
In this release, we have added the ability to compare to “concurrent controls” in the Platform Trial simulator, the frequentist calculation of conditional power, and we’ve added Fisher exact test and Bayesian Beta-Binomial analysis options to the dichotomous endpoint engines.
There is now the ability to load simulation results into the R package “Airship” that allows graphs to be created in an open and flexible way particularly for comparing designs. We’ve done a lot of work on the Bayesian dose escalation open enrollment option making the escalation decisions more consistent and transparent.
There are numerous small improvements and fixes and the start of implementing tooltips across the interface to make it more self-explanatory.
